Information Disclosure Statement (IDS) is an important and unique system in the US patent law. Specifically, according to the provisions of 37C.FR1.56 of the Implementing Rules of the US Patent Law, every person involved in the patent application (including inventors, assignees, agents, etc.) has the obligation to honestly inform the USPTO in the form of IDS before and during the application of the US patent application of important information related to the US invention application, in order to assist the USPTO in the review, including but not limited to prior art, publications, sales records, etc. This obligation continues until the patent authorization is announced or the patent application is withdrawn. If important prior art is not disclosed, the patent owner may not be able to implement the relevant patent.
IDS Submission Subject
US 37 CFR § 1.56 stipulates that everyone involved in the submission and examination of a patent application has the obligation to provide the USPTO with all information known to them that is substantially relevant to the patentability of the patent application, that is, the obligation to submit an IDS. Specifically, it includes:
- Each inventor named in the patent application
- Every patent attorney or patent agent who prepares or handles patent applications
- Any person who substantially participates in the preparation or prosecution of a patent application and is related to the inventor, applicant, assignee, or any person who is obligated to assign the patent application
IDS submission documents
According to the relevant provisions of the US Patent Law, the IDS documents that need to be submitted should at least include:
- (a) Prior Art Cited in Related Foreign Applications
- (b) Information Relating to or From Copending United States Patent Applications
- (c) Information From Related Litigation
- (d) Information Relating to Claims Copied From a Patent
In patent application practice, the IDS documents that need to be submitted to the USPTO include but are not limited to:
- (a) Prior art generated during the drafting and examination of family applications with the same priority, such as U.S. parent applications, divisional applications, continuation applications, and continuation-in-part applications, as well as family applications from other countries and regions outside the U.S. (including Chinese priority applications, PCT applications, European applications, Japanese applications, Korean applications, etc. that domestic applicants are familiar with), comparative documents cited by the Patent Office during the examination process, comparative documents submitted by the applicant to the Patent Office, and comparative documents mentioned in the application documents (such as background technology).
- (b) Prior art generated during the examination of applications that are technically related but have different priorities, and their family applications, for example, a series of applications in the same patent portfolio, and their family applications, comparative documents cited by the Patent Office during the examination process, comparative documents submitted by the applicant to the Patent Office, and comparative documents mentioned in the application documents (such as background art).
- (c) Prior art mentioned in the application documents of the U.S. patent application under review, especially the comparative documents mentioned in the background art.
- (d) prior art generated in relevant litigation and/or trial proceedings, such as prior art documents filed in court or at the patent office.
- (e) Others such as articles published by the inventor, prior art mentioned in the technical briefing, comparative documents retrieved by the inventor, patent agent and other search units, similar products of the applicant, and corresponding US applications that the US examiner believes may lead to duplicate authorization. If there is a case of copying claims from other patents, it is also necessary to indicate the source patent of the copied claims and the number of claims in the patent.
- For non-English prior art, if the English translation is not provided, the US examiner will usually not consider it, so it is also necessary to provide the corresponding English translation. The form of the English translation may include: English equivalent application (if any); full text translation in English (can be machine translation); English translation of relevant parts (mostly used for non-patent literature, can also be machine translation); English abstract.
- (f) Form PTO/SB/08: This form is required to list all relevant patent information (including US patents and non-US patents) and relevant non-patent literature information that is known.
- (g) Declaration: This is used by the applicant to provide relevant information to the USPTO, such as whether the disclosure document is more than 3 months old from the date the applicant became aware of it.
IDS submission time
During the U.S. patent application and grant process, IDS can be submitted at different stages:
- (a) Stage A: From the date of filing a U.S. patent application, or within three months of a PCT application entering the U.S. national phase, or before the issuance of the first non-final review opinion, or before the first review opinion after submitting a request for continued examination (RCE), an IDS may be submitted at this stage without paying any official fees or submitting any declaration.
- (b) Stage B: After the issuance of the first office action (Non-final OA), but before the issuance of the final office action (Final-OA), the grant notice, or any other action that may lead to the closure of the patent examination process. If a statement in accordance with 37 CFR § 1.97[1] is submitted and the disclosure document is within three months from the date of publication, no official fee is required, but a statement is required; if the disclosure document is more than three months from the date of publication, an IDS official fee is required.
- The statement required by 37 CFR § 1.97 may be either of the following:
- Each piece of information contained in this IDS was cited for the first time in a notification from a foreign patent office during the same foreign patent application process within three months before the submission of this IDS; or
- None of the information contained in this IDS has been cited in a foreign patent office notification during the prosecution of a foreign patent application family, and the person signing the declaration, after reasonable inquiry, is aware that no person specified in § 1.56(c) knew about it within the three months prior to the filing of this IDS.
- Considering the potential risks of using statement (2), applicants are generally advised to use statement (1).
- The statement required by 37 CFR § 1.97 may be either of the following:
- (c) Phase C: After the issuance of the Final-OA or the Authorization Notice, or after any action that may lead to the closure of the patent examination procedure, but before the payment of the authorization fee or on or before the date of payment of the authorization fee. If the IDS document is substantially related to the prior art (especially the X or Y category documents), in order to ensure the stability of the authorization, it is recommended to submit an RCE, and the IDS official fee and declaration need to be paid.
- (d) Stage D: Authorization fee has been paid, but authorization date has not yet been obtained. If an IDS is required at this stage, it is generally necessary to submit a request for withdrawal of authorization and an RCE request, and pay the official fee for withdrawal of authorization and the official fee for RCE. This method of restarting the examination procedure to submit an IDS is not only costly, but also time-consuming. At this time, it is necessary to submit a declaration, a request for withdrawal of authorization, a QPIDS and an RCE request, and pay the official fee for withdrawal, IDS and RCE.
- Since 2012, the USPTO has introduced a faster and lower-cost IDS submission pathway, QPIDS (Quick Path Information Disclosure Statement), which conditionally replaces the expensive and time-consuming RCE pathway.
- When submitting an IDS using QPIDS, you still need to submit a request to withdraw authorization and an RCE request, and pay the IDS official fee. However, the RCE submitted based on QPIDS is a "conditional" RCE: if the examiner does not find any IDS documents that need to be re-examined, the conditional RCE will not be initiated and the RCE official fee will be refunded; if the examiner finds that there are indeed IDS documents that need to be re-examined, the conditional RCE will be initiated, and in this case, the official fees other than the RCE official fee, such as the IDS official fee, will be refunded.
- From a practical point of view, the cases where UPSTO restarts the examination procedure after submitting IDS using QPIDS are rare. Therefore, submitting IDS using QPIDS after paying the authorization fee and before the authorization publication date is a quick and cost-saving method. However, QPIDS also has limitations. It is only applicable to the IDS documents that the applicant wants to submit to meet the time limit requirements specified in 37 CFR § 1.97. That is, if the applicant cannot submit a statement that complies with 37 CFR § 1.97, then the IDS cannot be submitted using QPIDS.
- (e) Stage E: After the patent authorization date. Generally, the IDS obligation ends after the patent certificate is issued. However, if the key IDS materials that should be submitted during the patent application process are not submitted, you can consider obtaining the opportunity to submit IDS through the supplementary examination procedure after obtaining the certificate, but you will need to pay a higher fee at this time.
We can clearly see the document and timing requirements of IDS through the table: 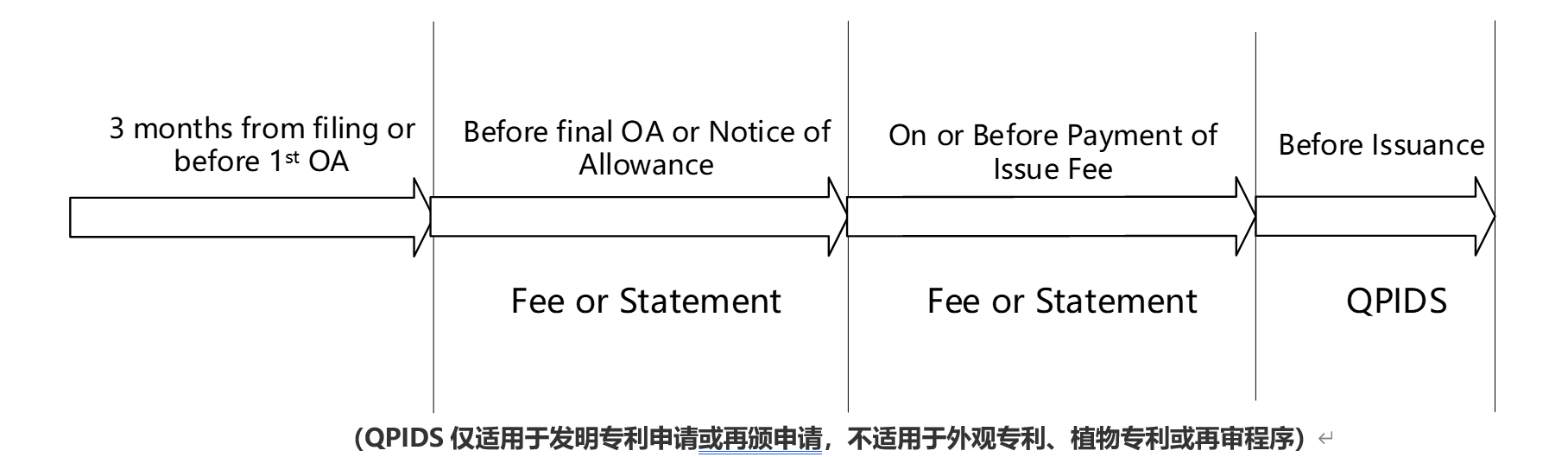
| IDS submission time | IDS Statement | Official Fees | |
| Phase 1 | Within 3 months from the date of filing in the U.S. or the date of entry of the PCT application into the U.S. national phase, or before the first office opinion (Non-Final OA); or before the first office opinion after the RCE request | none | none |
| Phase 2 | After the first office action, but before Final OA, Notice of Allowance, or any action leading to the closure of the patent examination process | Yes (declared within 3 months from the date of first learning of the IDS information) | none |
| have |
| ||
| Phase 3 | After the Final, Grant Notice or any action that may lead to the closure of the patent examination procedure, but before or on the day of payment of the grant fee | have |
|
| Phase 4 | After paying the authorization fee and before the authorization announcement | have | 3000.00USD (large entity) + RCE official fee |
Note: The obligation to file an IDS continues until the patent application is abandoned or the patent is issued. Even if you receive a notice of allowance, it does not mean that the obligation to file an IDS is over. You must wait until you pay the authorization fee and receive the issue notification.
The USPTO will implement new patent application fee standards on January 19, 2025, which will establish a new tiered fee structure for information disclosure statements, or IDS. According to the new regulations:
- If the cumulative number of IDS submitted exceeds 50, a fee of US$200 is required
- If the cumulative number of IDS submitted exceeds 100, a fee of US$500 is required
- If the cumulative number of IDS submitted exceeds 200, a fee of US$800 is required
- The applicant must also provide a written statement describing the size of the IDS fee submission and the status of the fee payment.
联系咨询
 | Get exact prices For the country / regionE-mail: mail@yezhimaip.com |

